As a core member of the ‘CI’ team, I helped to monitor & prioritise, identify root-causes, and ultimately resolve a number of reliability issues with the current product range. I also lead a cost-reduction project, and contributed to a range of process improvements.
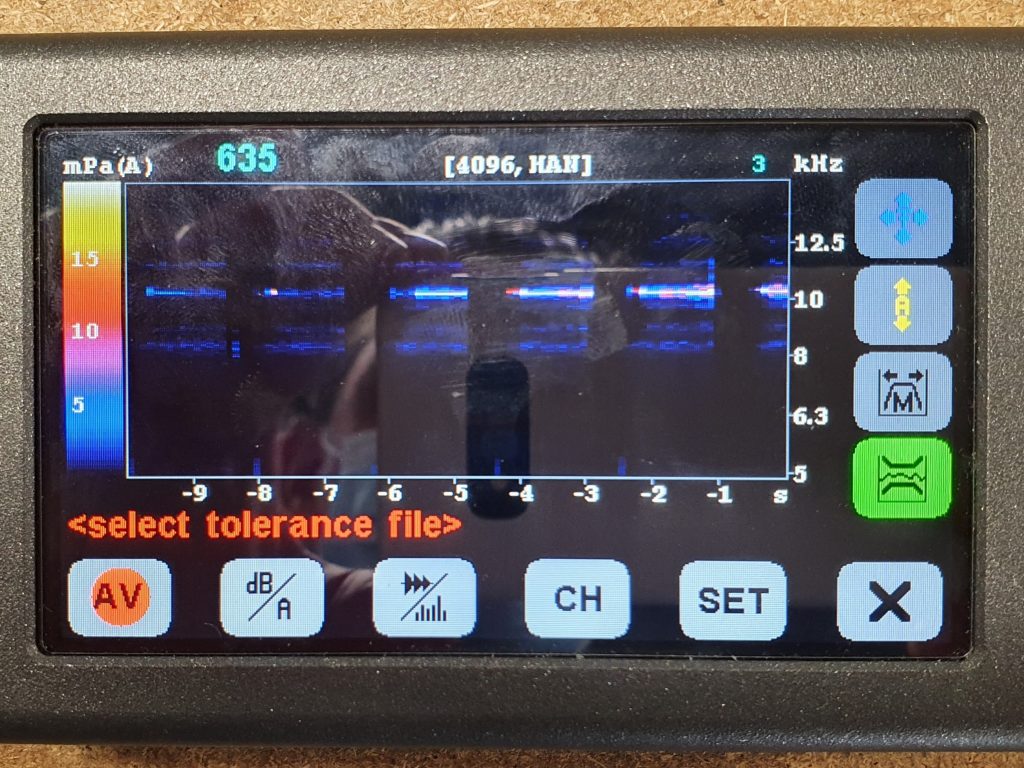
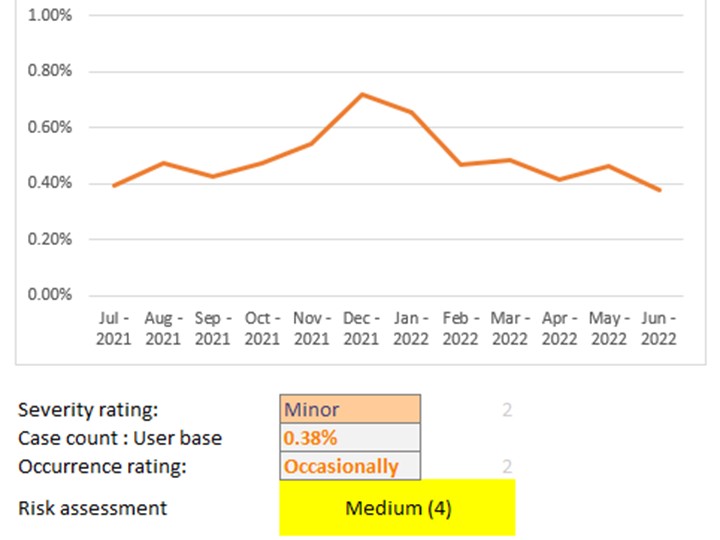
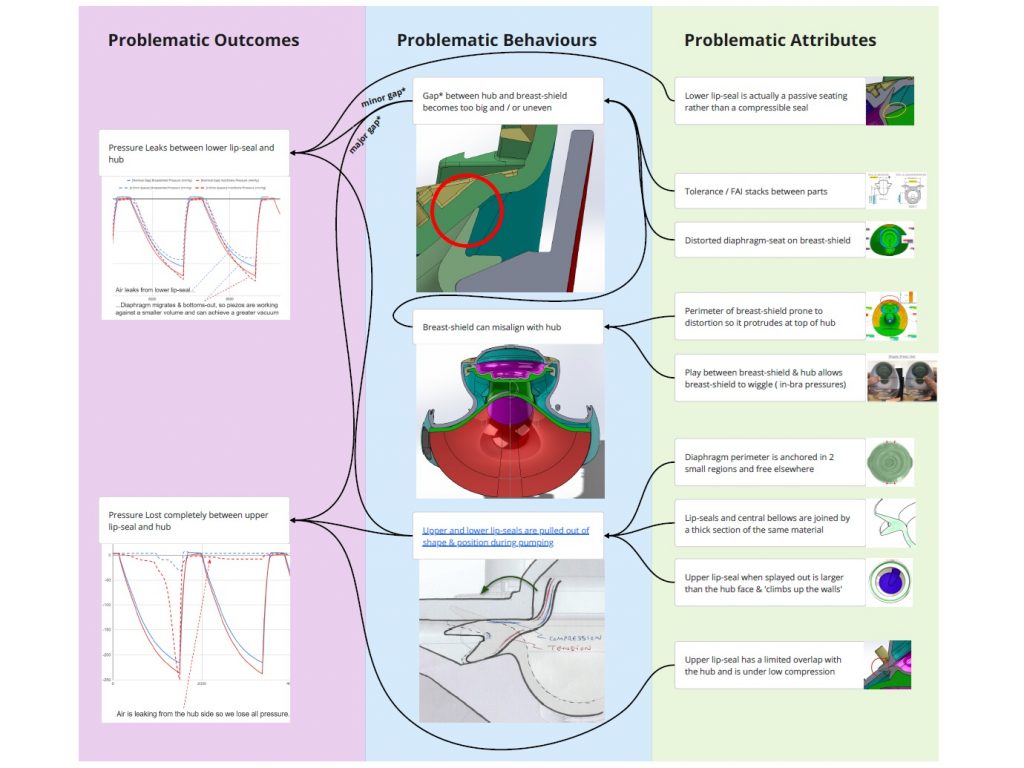
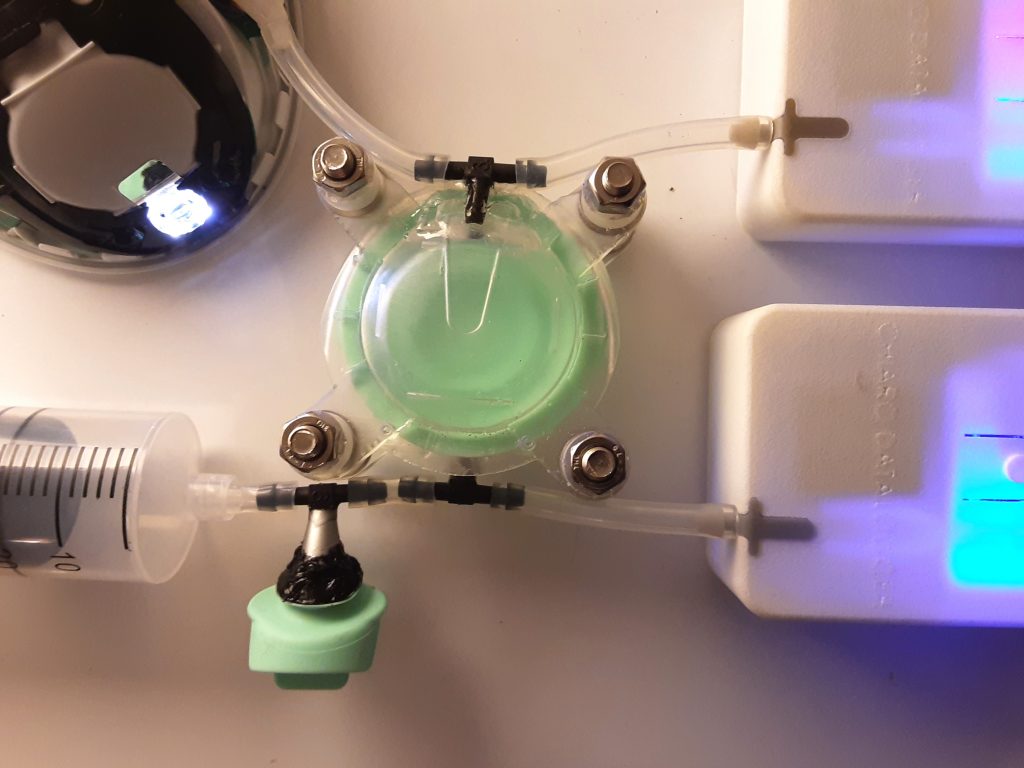
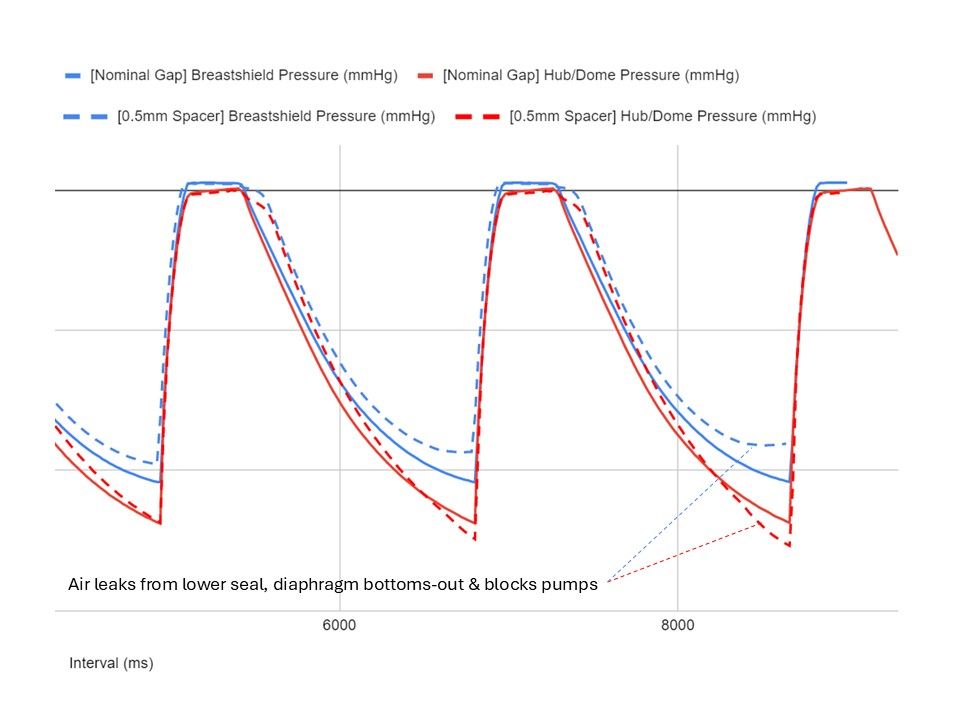
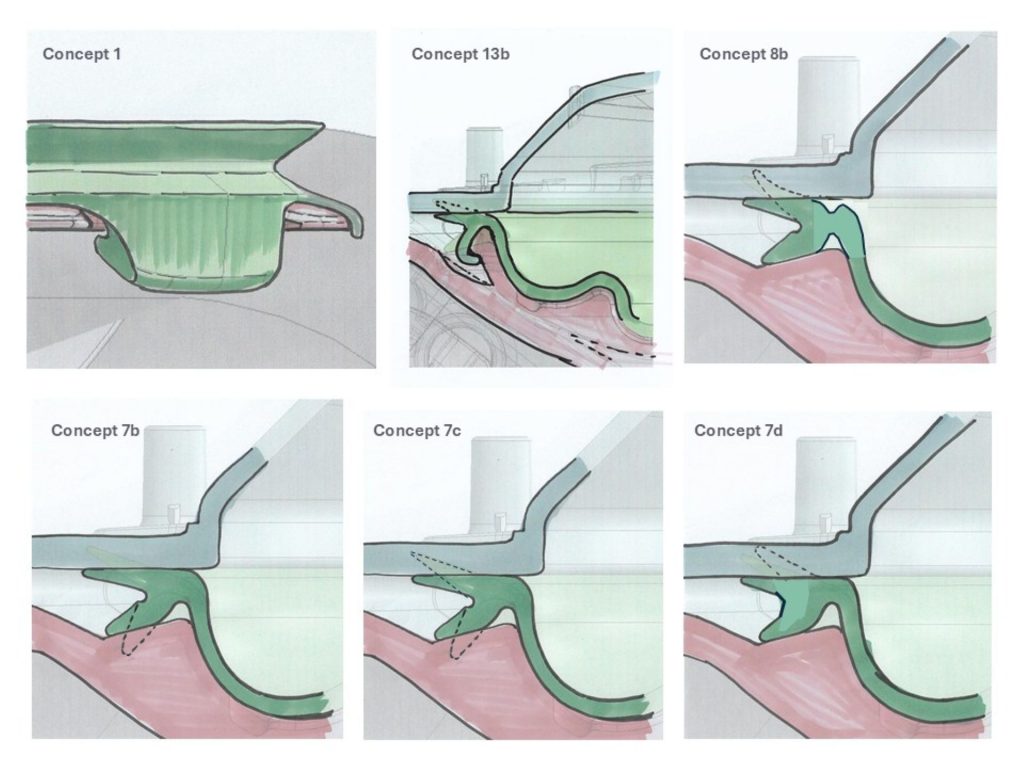
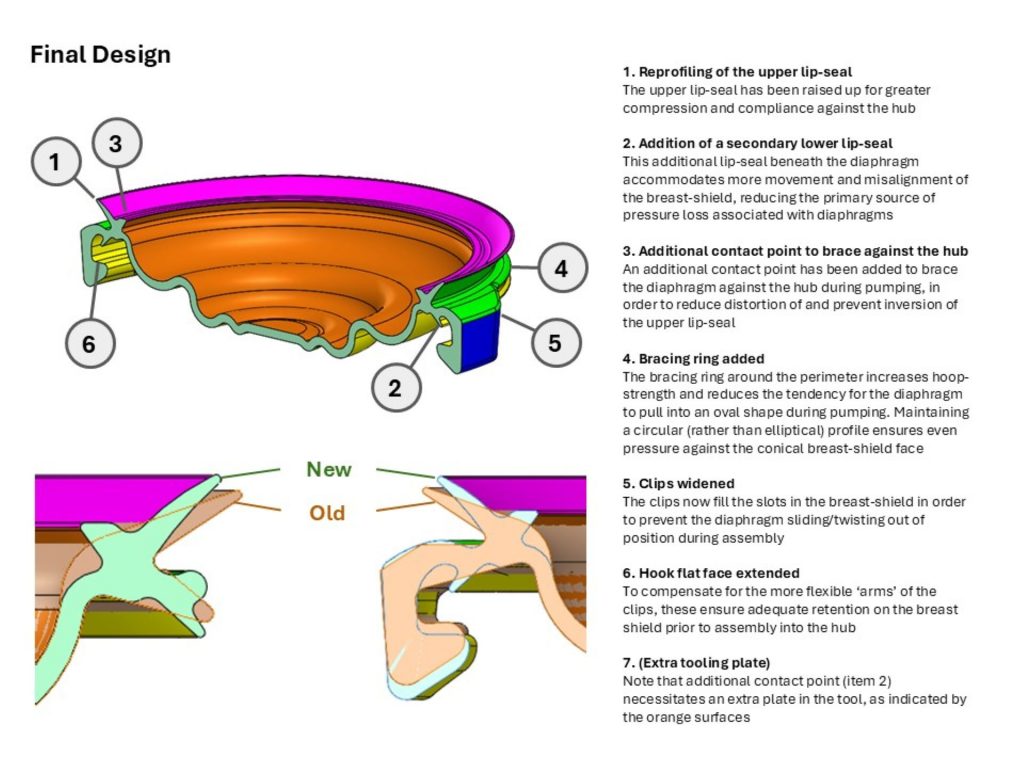
I undertook experiments and fault-tree analysis to understand the complex range of dynamic factors which were causing intermittent pressure loss on a breast pump. I designed and 3D-printed a pressure-testing rig to isolate the most problematic sealing features; this was then used to test a range of prototype seals of my design in order to verify the improvements. I collaborated closely with our Research team to define a user study and assess the outcomes; this validated the new design. I also liaised directly with the seal manufacturer to obtain quotes and to sign-off the DFM & tool layout, before then managing the change into mass production, releasing my new drawings and updated BOM via our ECO process. Our monitoring of post-market issues subsequently showed a significant drop in complaints related to pressure loss.

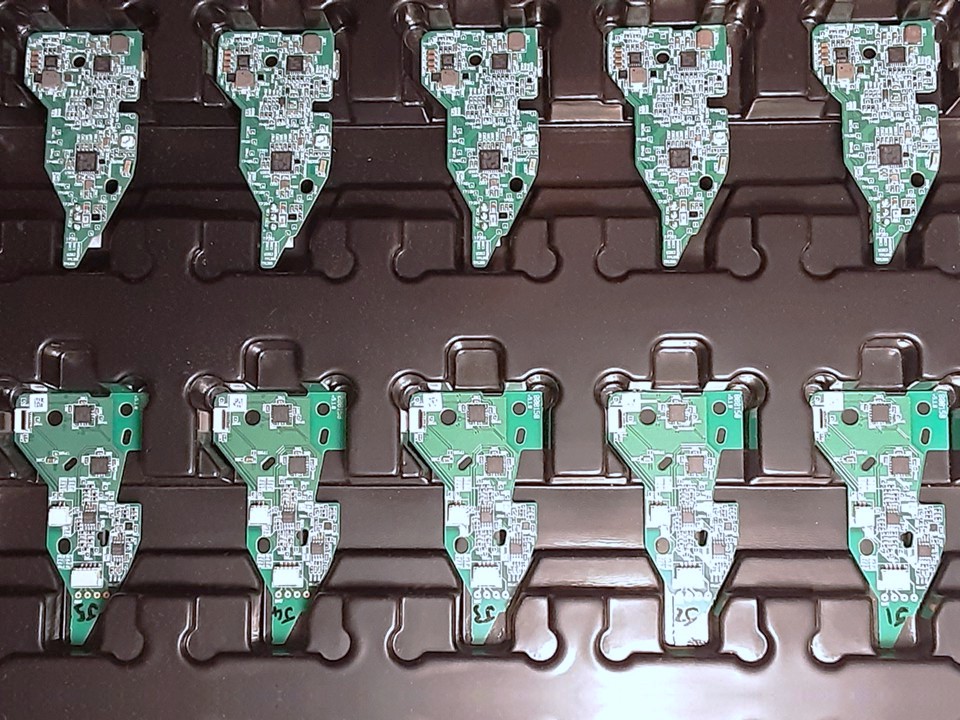
I design-managed a significant cost-reduction project through to mass production. This involved close collaboration with our electronics team and contract manufacturer to remove a redundant sensor from a PCBA. It also impacted the pneumatic system, so I designed, tested and implemented tubing changes which brought further cost savings. Again, I was heavily involved in defining and assessing the outcomes of multiple verification and validation tests, updating various drawings and specifications, and managing the change through our ECO process.

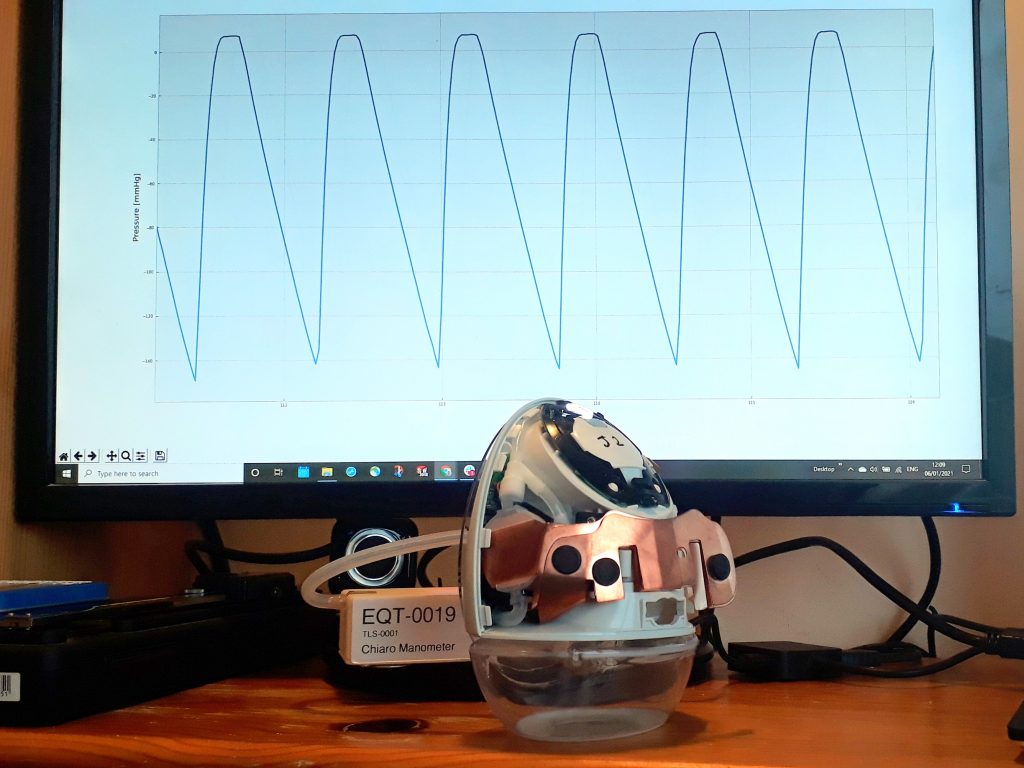
I worked with an electronics contractor as we sought to resolve an intermittent Bluetooth connection issue on a wearable medical device, which my investigations had already isolated to the antenna. I helped create a body-simulating test-rig and made an antenna bending rig, then hand-modified and tested a range of devices in order to assess proposed antenna changes via signal-strength testing. I also worked with our research team to define and run user-studies, then arranged EMC testing of modified samples, and liaised with the contract manufacturer to prepare for implementation.
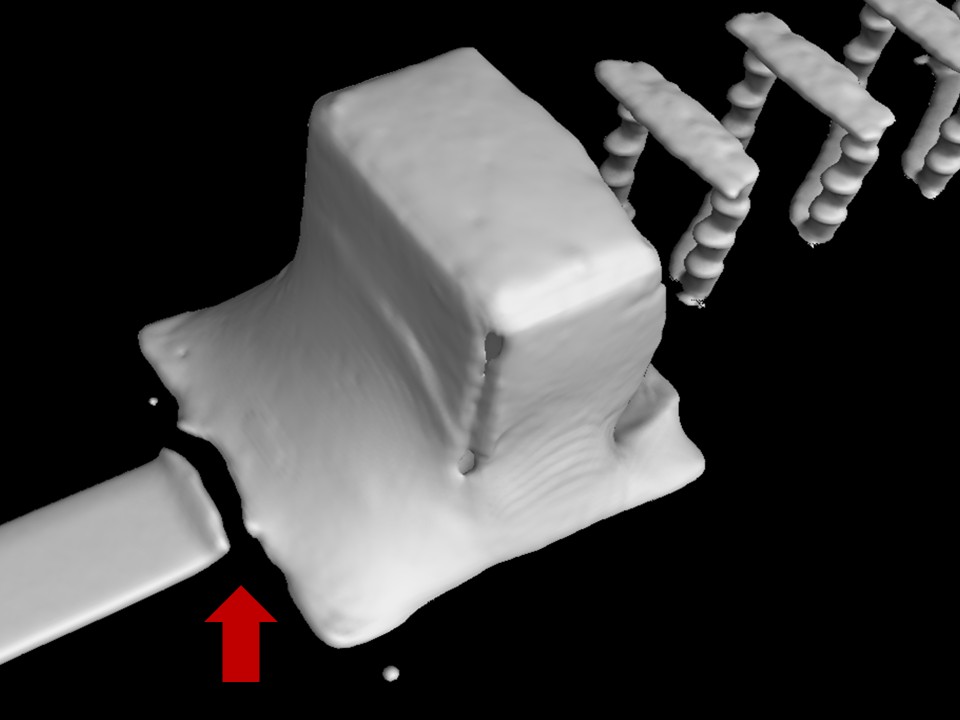
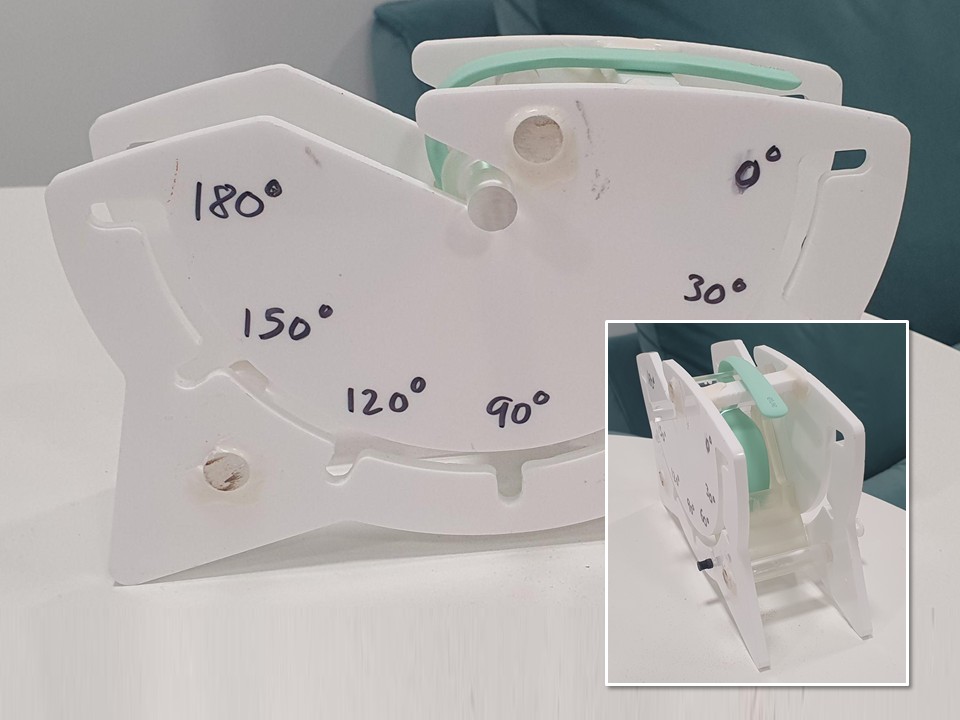

My other notable ‘CI’ projects include implementing upgrades to USB cables to resolve durability issues, collaborating with our pump component supplier to reduce noise, and working with the quality team to refine and help run a new complaint-monitoring & investigation process which directly contributed to Elvie obtaining certification to ISO 13485 (Medical Device QMS).